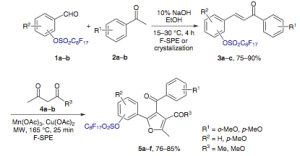
I have to imagine that when an organic chemist really digs into an article, he/she thinks what they could use it for, and how would they have done it differently…..I know I just can’t help myself, and it should be seen as a complement to the author. I recently read through a nice paper (Synlett 2011 pdf author reprint) on the preparation of tetra-substituted furans — cyclization (Mn and Cu accelerated) through a chalcone derivive with a pending fluorous linker ( a term that gets debated a bit). The cool thing is that the linker can be set up to help purification, aid in additional steps including cleavage or transformation to additional analogs.
The following scheme is shown to give you an overall idea of the strategy. The fluorous linker is now set up to undergo typical transformations such as Suzukis and Hartwig-Buchwald aminations (like a triflate would) or simply cleaved at some point following changes in the groups on the furan. There certainly is a lot of possiblities (aryl and subst alkyl boronates, slew of amines and anilines, etc).
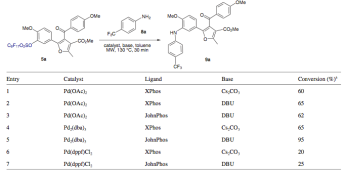
OK so now the part I found both interesting but also where I would want to do things differently….for the Suzuki, there are a limited number of aryl boronic acids used — sure there is a significant rate acceleration using the mw -hours to minutes, but not enough diversity to peak my interest in what can be done. So I always wonder why — if the compounds are so interesting, why not expand to alkyl, heteroayl, etc? Is it because they are using a single-mode microwave and therefore at the mercy of doing a reaction at a time sequentially? For me it’s like you want to take away the benefit you got by using the alternate technology in the first place….not for me at least. The second thing the group did was an optimization of reaction conditions for the Buchwald couplings — solvent — toluene, base, catalyst, ligand but kept the same temperature. I felt a little let down at the part of the movie……you have heard me talk about microwave SRC technology — this is where you could screen solvent, base catalyst, amines, ligands all at the same time [this is is saving weeks worth of work]– could even change the functionality into an ester or ketone under a CO atmosphere……I would go crazy to have a set up like that. Maybe that’s my former med chem days leaking into my reading.
Look through the optmization — all makes sense, but at 30 min a pop, which to me is too valuable a timeline not to be done simultaneously, there is a point of no return or which one simply doesn’t do the next necessary reaction. At any rate, a really cool scheme to make some compounds of interest…and something I would jump at for these type of furans. Keep in mind when a choice is made to use mw for libraries or optimization, there is a strategy that comes into play as well. Just choosing the microwave doesn’t make it efficient unless you think about the entire goal. Enjoy the article. Happy Reading!