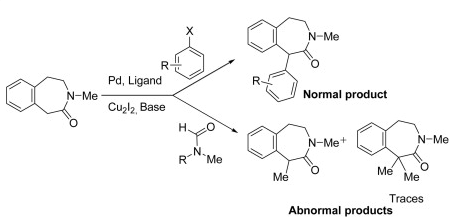
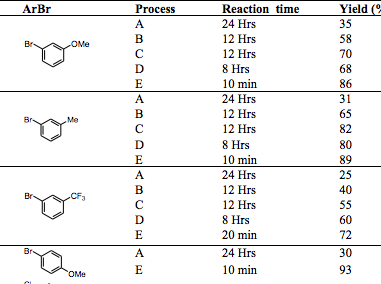
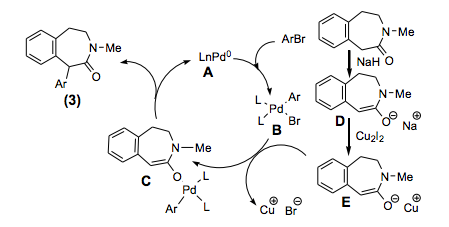
I came across an interesting article on alpha arylation of a 3-benzazepin-2-one, which is a particularly sluggish reaction (TL 2013). First thing is first, the formation of a traditional amide enolate with n-BuLi doesn’t effectively alkylate with simple substrates and the use of NaH was needed as the base to obtain the desired reaction (this was back in 1979), which was not going to be a good starting point for arylation. Unfortunately, the addition of Pd into the cycle did not produce any of the desired product so the base strength was a consideration as an issue. The addition of Cu2I2 from Li and Na amide enolates provided the first successful formation of the desired material with heat helping the reaction along (and effectively forming a Cu-amide enolate that can transmetallate). A switch from Li to Na improved the process, but examining solvent and the best Pd source (Pd2dba3) for the combination improved the process. Once the addition of microwave heating with NaH (DMF:Dioxane 1:5) with stoichiometric Cu2I2 effectively entered the Pd Cycle to provide high yields in short reaction times (10-20 minutes from 12 hours)…..and the dependency on EWG, electron-neutral or EDG was negligible.
The table shows the improvement in moving from n-BuLi, Cu2I2 to NaH, Cu2I2 to microwave heating with method E.
The entire cycle where the group finally worked out the entire process is shown below. Interesting backbone that shows potential for extending what can be performed if the Me on the nitrogen is replaced and the lactam further functionalized as a handle. Happy Reading!


Reblogged this on ORGANIC CHEMISTRY SELECT.