The preparation of nanoparticles with microwave energy has developed extensively over the last 5 years and I have posted examples of this development in the recent past (Review of Inorganic Microwave Approaches and Microwaves in Nanoparticle Synthesis: Fundamentals and Applications). But a recent publication (Sustainable Chemical Processes 2014) utilizing specific properties of nanoparticles with a magnetic core (MNPs) was particularly intriguing. In addition to the ability to adjust or tune different structures, compositions and morphologies, these structures offer an opportunity to run cleaner reactions with easy separation from reaction media in additional to reaction media to be used in place of typical organic solvents –so they satisfy some of the core fundamental ideas in a “Green Process” and lend themselves to enabling technologies as Ultrasound, Microwave and Mechanochemical mixing. I will show a couple of examples, but this is something everyone should take a look at as an alternative to existing strategies. The article discusses several areas where these core structures can be changed depending on the transformation needed.
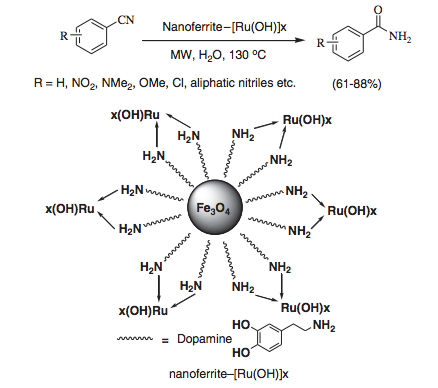
In the example below, we see a hydration of a cyanobenzene to the corresponding amide with a nano-ferrite magnetic core with an Ru-OH exterior. At the end of the reaction, the catalyst is simply removed with a magnet on the exterior of the reaction vessel and the amide crystallizes on cooling.
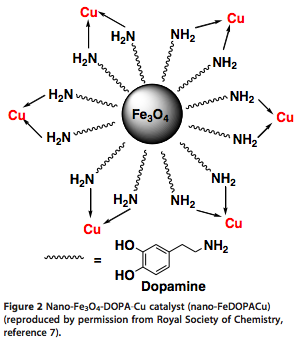
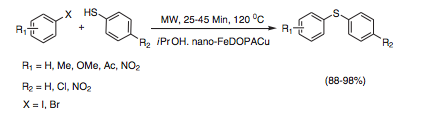
With many ways to modify the central core, the catalyst shown below was used in a C-S coupling reaction with aryl halides and thiophenols under microwave heating in high yields in 25-45 min.
As you can see I view this as an opportunistic publication — one where it is merely putting a small positioning out there and chemists can mold the shape of the technology. Happy reading!