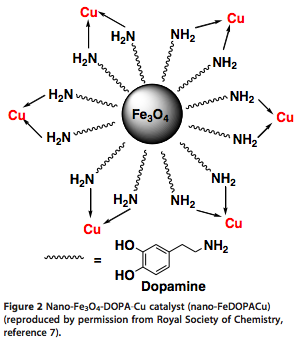
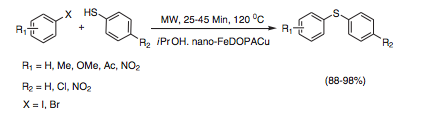
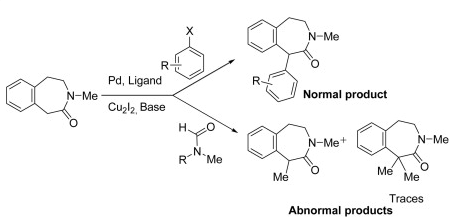
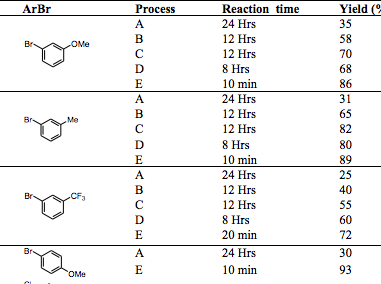
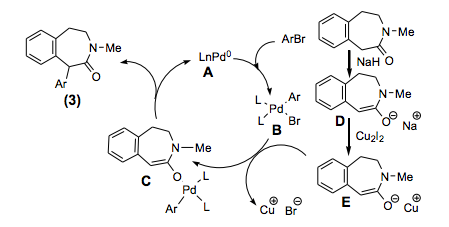
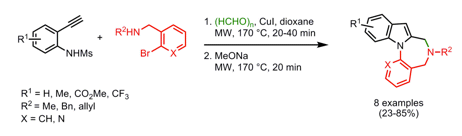
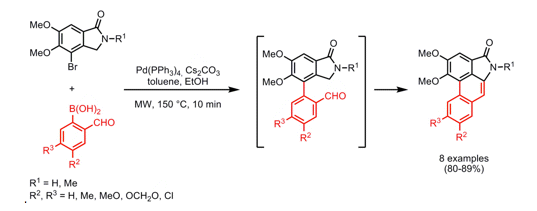
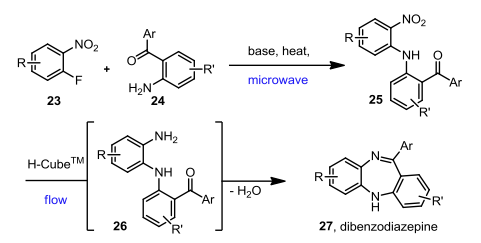
I often wander how some chemists come up with their retrosynthetic approach or if it is simply that they have seen so many reactions that visually it all comes together. I spent most of my career in the Nitrogen non-aromatic and heteroaromatic world, so when I see a fused pyridine, quinoline, pyrrole, isoxazole I have a large library in my head on things done….condensations, Pd-mediated events….but I have to admit I never see these in my head, and that is a way to extrude N2 in the formation of a pyridine ring.
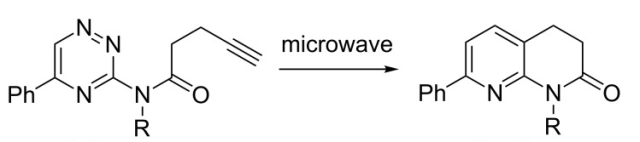
The reaction scheme below (Beilstein JOC 2014) utilizes a 1,2,4-triazine in a thermal DA(INV) reaction in the construction of a 3,4-dihydronaphthyridone system. This not only serves as a masked construction of the the fused pyridine (naphthyidone), but also serves as a way to substitute depending on the group on the alkyne or something in place of the Ph group on the triazine. The microwave conditions: 1 h, chlorobenzene, 220C to provide a route into a library of 1,5,7-substitution patterns around the ring.
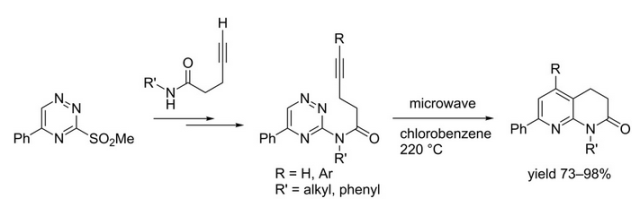
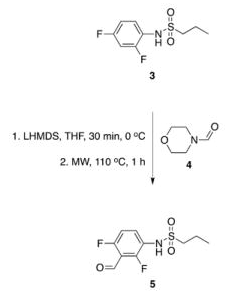
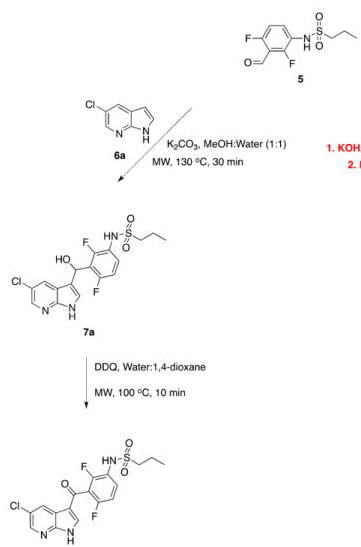
The forward scheme into the intermediates is also captured below:
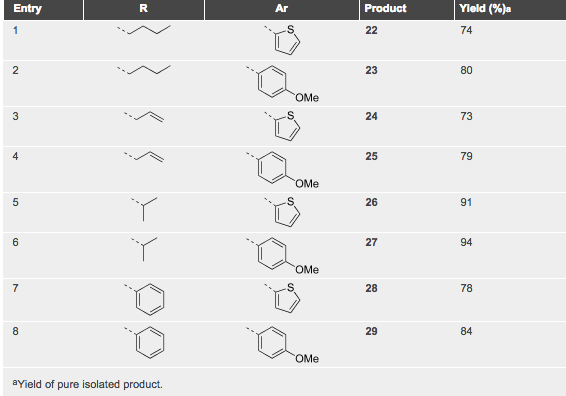
A table of examples illustrates the use of the triazine in the DA process — clearly open to a number of changes for this transformation.
As I mentioned — it impresses me that some chemists can think backwards in these structures — I get it, because this strategy is not new, but it just seems like when I look at a pyridine ring, I can’t visualize the triazine prior — For additional examples of inverse-electron demand hetero diels-alder reactions see the following paper in the construction of N-heteroaromatics (Chem Sci Rev 2013). Happy Reading!