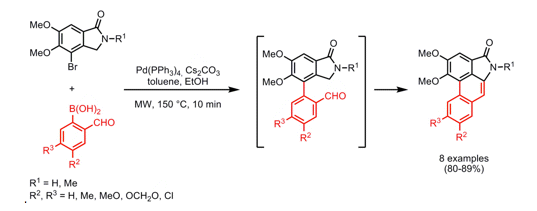
An elegant approach to a variety of Aristolactams was reported in Org Lett 2008, taking advantage of alpha-formylaromatic boronic acids and an appropriately substituted lactam. As illustrated below, initial coupling with the bromide on the advanced lactam with a variety of aryl and heteroaryl boronic acids provides a the bis-aryl coupled product which undergoes a simple aldol condensation to close the ring in a one-pot format under microwave heating at 150C in 10 minutes…..providing a route to natural aristolactams as well as an opportunity to study substitution patterns around the phenanthrene or heteraromatic fused rings.

-
Recent Posts
- Effective use of an inverse demand Diels-Alder: the microwave way November 20, 2014
- Combination of MW and Nanocatalysts with Magnetic Core: Greener Approach to Synthesis November 11, 2014
- Alpha-arylation of 3-benzazepin-2-ones with microwave heating November 10, 2014
- Domino 3 Component Microwave: Mannich, Indole formation, N-Arylation November 7, 2014
- Aristolactams: One-pot Miyaura-Suzuki/Aldol cascade microwave method November 6, 2014
Chemistry Resources
Journals Of Interest
Labs Utilizing Microwave Strategies
- Albert Stiegman – Florida State University
- Dr. Nicholas Leadbeater – University of Connecticut
- Florida State University – Gregory Dudley
- FMF-Nanolab at the University Freiburg
- Idaho State University – Joshua Pak
- O. Kappe – University of Graz
- UCLM – Microwave and Sustainable Organic Chemistry
- University of Kansas – Paul Hanson
- University of Turin – Giancarlo Cravotto
- York University – Michael Organ
Links
- All Things Metathesis
- CENtral Science – The Haystack
- Chemical Crystallinity
- Chemistry Cascade
- Chemjobber
- Curly Arrow
- Heterocylist
- In The Pipeline
- Nanoall
- Naturalproductman
- NNNS chemistry blog
- Organic Times Syndicate
- Organometallic Chemistry
- SynArchive: An organic synthesis archive
- Synthesizing Ideas
- Syntheticnature
- The Free Radical
- The Heterocyclist
- The Organic Chemistry Blog (The BOC)
Microwave Synthesis Books
- Laboratory Experiments using Microwave Heating
- Microwave Heating as a Tool for Sustainable Chemistry
- Microwave in Organic Synthesis
- Microwave-assisted Organic Synthesis: A Green Chemical Approach
- Microwave-Enhanced Polymer Chemistry and Technology
- Microwaves in Nanoparticle Synthesis: Fundamentals and Applications
- Microwaves in Organic and Medicinal Chemistry (Methods and Principles in Medicinal Chemistry)
News Wire
Reviews
Websites of Interest
Recent Comments
DR ANTHONY MELVIN CR… on Domino 3 Component Microwave:… DR ANTHONY MELVIN CR… on Alpha-arylation of 3-benzazepi… Combination of MW an… on New Review for Inorganic micro…