The words microwave heating and carbohydrates are usually used together to roll of the tongue when talking about microwave technology. There have been a number of times, I have worked with carbohydrate researchers who insist that microwaves destroy and don’t have a good place with their chemistry — but give it some thought, this doesn’t mean we are going to heat things to 100-200C, moving from room temp to 50C is a major change in the energy of the system. OK- enough on that: a contemporary review on microwave heating in turning carbohydrates in heterocycles (is this like going from wine to water, lol!) has just been published by Venerdando Pistara at the Dept of Pharmacy – University of Catania, Italy (Current Organic Chemistry 2014, covering the major work accomplished in the last 4-5 years…..it’s a good review and opened my eyes to some interesting ways to use carbohydrates as part of the synthetic arsenal.
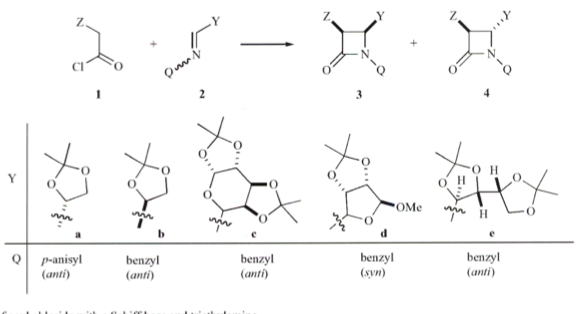
Let’s start off easy with some simple methodology in the formation of optically active cis-b-lactams. In using an acid chloride and TEA, a mixture of cis/trans b-lactams with a low level of microwave heating — and higher temperature didn’t help. Moving over to a Schiff base of the carboydrate substrate provided the cis-b-lactams and could be performed on a reasonably large scale in a commercial microwave at 800W of power for 3 minutes.
Now moving over to the formation of heterocycles. Generation of optically active pyrazoles has been in the literature a fair bit lately. Utilizing a 2-formyl glycol and hydrazines, several optically active advanced pyrazoles were provided under microwave heating (TL 2004).
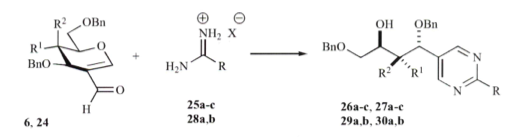
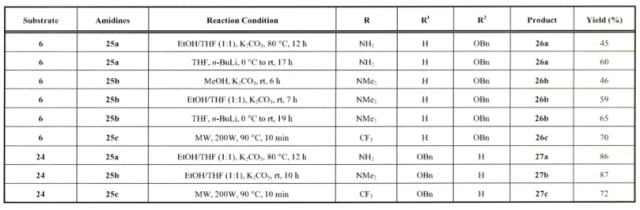
If we take this approach as a comparison to conventional heating – and exchange the pyrazole with a pyrimidine (from 2-formyl glycals and amidines, you will notice the reaction optimization illustrates the shortened reaction time utilizing microwave heating (TL 2008).
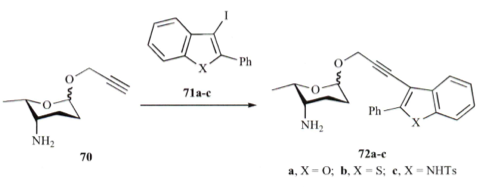
Pd-mediated microwave couplings can also be performed on carbohydrate substrates as shown in the example of an acetylene with a b-amino glycoside substrate in a Sonogashira coupling (Carbohyd. Res. 2010)
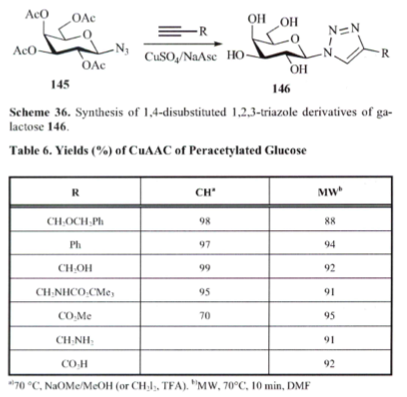
Additional examples of transformations highlighted in the literature (Click chemistry) can be found with the formation of a linked carbohydrate triazoles from galactose azides in the presence of a variety of acetylenes under microwave heating, and opening the ability to substitute at the C-1 and C-6 position (Biorg Med Chem 2010).
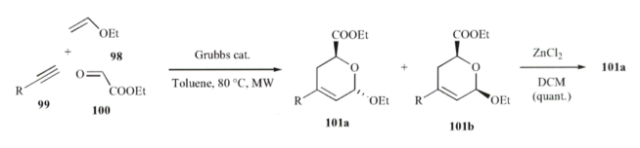
Stepping away from from having the sugar backbone in place, a mulitcomponent RCM-HDAR with 2nd generation Grubb’s catalyst was an excellent entry into the construction of the pyran ring without the need for protecting group chemistry (Carbohyd Res 2009) in toluene at 80C under microwave heating.
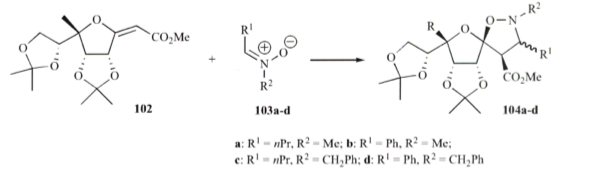
Several additional examples of linking, ring construction and annulated ring systems are covered throughout the remainder of the review, and if you have any interest in sugar chemistry or the possibility of using the backbone as a handle then you need to understand some of the strategies laid out in this review — and see that microwave technology is been used extensively. So my last example is the formation of a spiro substituted sugar from a nitrone-sugar olefin cycloaddition. Using exo-glycal and a variety of nitrones, the spiro-isoxazolidines could be formed under microwave heating (Lett Org Chem 2005) — of course this has been applied to the 6-membered ring variation as well. Enjoy the rest of the review — Happy Reading!