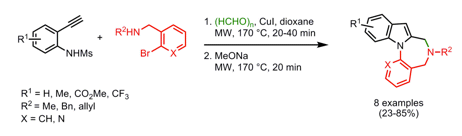
I have been reading through cyclocondensations and multi-step routes to fused ring systems and came across a highlight from Doris Dallinger’s post on the Organic Chemistry Portal. Although a bit older I have always found domino multi-component reactions have a penchant for the dramatic and this example (OL 2008) is no exception. This group, out of Kyoto, utilized a Cu(I) catalyzed 3-component microwave reaction sequence, starting with a Mannich, followed with an indole ring formation at 170C for 20 min. Addition of NaOMe and re-heating in the microwave for an additional 20 min at 170C deprotected and N-Arylated the Indole nitrogen for a short route to mixed-1,4 diazepines. Solvent studies and catalyst loadings were optimized to show dioxane and 2.5%CuI for the protocol. In addition to the benzene ring on the lower portion of the scaffold, additional heterocycles were used to broaden the availability of fused rings….which would tell me that the amine, left-hand or indole substitution and the lower ring system can be varied to include a number of different features as well as additional space to consider from a med chem point of view.
If the ring system looks slightly familiar or the subject matter, I have posted an indole based domino sequence in the past and if it just happens to be that day, have a look. Happy Reading!