This is an area that you don’t typically read about when thinking about microwave technology — a lithiation followed the reaction of a subsequent electrophile with heat, and that it usually because the initial deprotonation is performed at low temperature….in fact these techniques are not adequately discussed in the heavy hitting books that we have come to rely on in the last 5-10 years…some due to the technology and some because the percentage of reactions hasn’t taken its’ place in the column, sitting way down the list from couplings, condensations, cycloadditions, etc. If one thinks about it intuitively, typical deprotonations are performed in non-polar non-protic solvents that are not generally used in microwave heating — but give it some more thought……anions formed, higher-ionic content following the deprotonation is an excellent way to spice up your non-absorbing solvent media.
Well I am simply going to point to a couple of examples so that it at least is a potential strategy to consider.
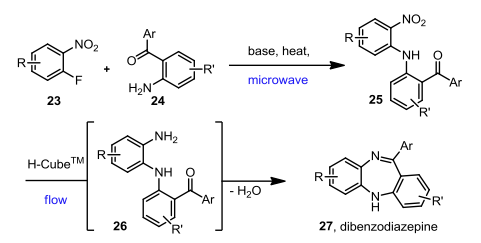
The first report comes from Ley and Baxendale — and their paper (Molecules 2014) is on the topic of flow chemistry to form different pharmacophore scaffolds….and in the process, they needed to deprotonate an aniline to react with a fluoroarene with subsequent heating for the SNAR to be a useful process. Although, this was an excellent way to make an advanced intermediate, it helped the group transfer the technology over to a flow method — back to the microwave. As mentioned earlier, most chemists don’t do their microwave chemistry this way, but it should be food for thought — do you know enough about the stability of the lithiated species — is it stable at 0C, can the electrophile be present during the lithiation and once the anion or lithiated species is formed, can it be heated?
The scheme below shows that in fact the process to form the desired nitro diaryl amine could be made efficiently in a microwave with the lithiation step at RT in the presence of a 1:1 mixture of aniline:fluoroarene and subsequently heated in a microwave for 30 min at 100C…….there are plenty of ways to use this strategy, but it’s just underutilized.
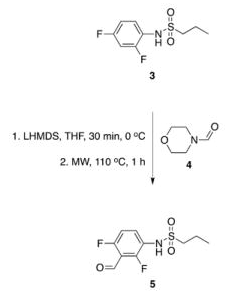
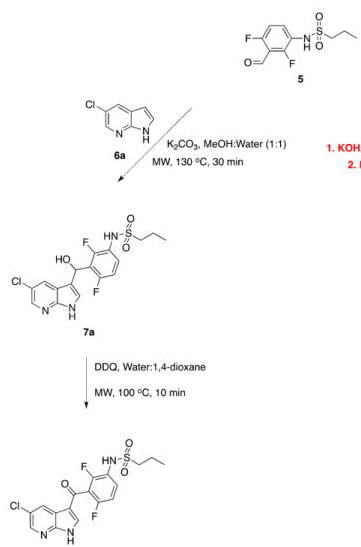
The next example (TL 2012) is the typical grueling process of decision making that one undertakes when moving from a reaction where you would traditionally deprotonate, and in this case, there is more than one lithiation…..let me make it more complicated — they had already done this in prior publications with LDA and multiple hours and multiple temperatures to provide compound 5 (Full disclosure — I wasn’t looking for a lithiation — this group was making BRAF inhibitors, and were comparing sorafenib (fondness of my former Bayer days). So in this example, the lithiation was changes over to LHDMS at 0C for 30 min, followed by the addition of the formyl-morpholine and heated to 100C in the microwave to produce the desired advanced intermediate 5, which was split into two divergent pathways to final compounds. The scheme below shows the final process for the synthesis of one of the final compounds. Notice the additional microwave steps to the final compound. 🙂 Happy Reading!