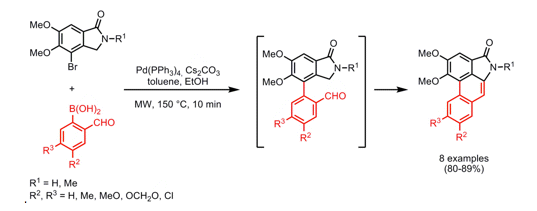
An elegant approach to a variety of Aristolactams was reported in Org Lett 2008, taking advantage of alpha-formylaromatic boronic acids and an appropriately substituted lactam. As illustrated below, initial coupling with the bromide on the advanced lactam with a variety of aryl and heteroaryl boronic acids provides a the bis-aryl coupled product which undergoes a simple aldol condensation to close the ring in a one-pot format under microwave heating at 150C in 10 minutes…..providing a route to natural aristolactams as well as an opportunity to study substitution patterns around the phenanthrene or heteraromatic fused rings.
Tag Archive: Suzuki-Miyaura
After coming across the phase separation strategy for preparing medium to large macrocycles with a 1,3-diyne moiety, I thought about the holes in my background in this area — which is a bit strange considering I started my career at Abbott and with all the Erythromycin or macrolide chemistry performed there in general one would think I would have been up on it…alas, about as much as not finding Clarithromycin internally (not really a parting shot, but if the resident experts miss a billion dollar drug how am I expected to keep up — knowing the stories it is easy to miss). 😉
Usually the intro for this topic starts with clinically relevant and natural macrocyclic antibiotics — lots of them, but there is so much more from branching polycyclic peptides to the antifungal and antiparastics adding to the structural diversity. I certainly remember when the epothilone synthetic strategies came out initially — still a hot topic with all the relavance for cancer targets.
If you want a good current review of synthetic methodologies toward macrocycles — and their relevance to drug discovery — turn to Molecules 2013. Dianqing Sun does an excellent job of breaking down each of the classes of macrocyles, strategies in their synthetic pathway and their relevance in drug discovery and natural product research. For me — haha, I am so selfish…I found some examples where the microwave has gone unexplored in the “macrocycle” world. I have touched on ring closing metathesis (RCM) in the microwave for all of the obvious benefits and at the risk of turning on that broken record — nearly all of the ring-closing steps INCLUDE a heating as a critical component. However as you can imagine — there was some time dedicated to metal-mediated C-C and C-O couplings OR, hmmm, maybe a Buchwald-Hartwig cyclic amination.
I have included a few of the relevant references below:
Lépine, R.; Zhu, J. Microwave-Assisted Intramolecular Suzuki-Miyaura Reaction to Macrocycle, a Concise Asymmetric Total Synthesis of Biphenomycin B. Org. Lett. 2005, 7, 2981–2984.
Su, Q.; Beeler, A.B.; Lobkovsky, E.; Porco, J.A.; Panek, J.S. Stereochemical Diversity through Cyclodimerization: Synthesis of Polyketide-like Macrodiolides. Org. Lett. 2003, 5, 2149–2152.
Pérez-Balado, C.; Nebbioso, A.; Rodríguez-Graña, P.; Minichiello, A.; Miceli, M.; Altucci, L.; de Lera, Á.R. Bispyridinium Dienes: Histone Deacetylase Inhibitors with Selective Activities. J. Med. Chem. 2007, 50, 2497–2505.
Afonso, A.; Feliu, L.; Planas, M. Solid-phase synthesis of biaryl cyclic peptides by borylation and microwave-assisted intramolecular Suzuki-Miyaura reaction. Tetrahedron 2011, 67, 2238–2245.
Nnanabu, E.; Burgess, K. Cyclic Semipeptoids: Peptoid-Organic Hybrid Macrocycles. Org. Lett. 2006, 8, 1259–1262.
Bedard, A.-C.; Collins, S.K. Microwave accelerated Glaser-Hay macrocyclizations at high concentrations. Chem. Commun. 2012, 48, 6420–6422.
Dong, H.; Limberakis, C.; Liras, S.; Price, D.; James, K. Peptidic macrocyclization via palladium-catalyzed chemoselective indole C-2 arylation. Chem. Commun. 2012, 48, 11644–11646.
For those of us who enjoy the visual schemes:
I particularly like the next reaction — an intramolecular Ullmann approach (plenty of these in my nightmares — I think I probably ran a thousand of these reactions). Go ahead and look through SciFinder for a macrocycle with phenoxy-alkyl or aryl and you are set up nicely for this type of ring closure (e.g. Hisutellone B)
For a detailed account of this approach compared to much more traditional sealed tube and conventional thermal heating check out Tett. Lett. 2011, 52, 4570-4574.
Enjoy the article. Hopefully it makes you think about additional ways to improve this area of research.