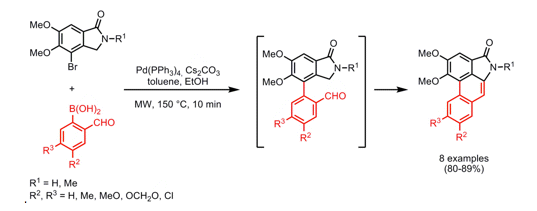
An elegant approach to a variety of Aristolactams was reported in Org Lett 2008, taking advantage of alpha-formylaromatic boronic acids and an appropriately substituted lactam. As illustrated below, initial coupling with the bromide on the advanced lactam with a variety of aryl and heteroaryl boronic acids provides a the bis-aryl coupled product which undergoes a simple aldol condensation to close the ring in a one-pot format under microwave heating at 150C in 10 minutes…..providing a route to natural aristolactams as well as an opportunity to study substitution patterns around the phenanthrene or heteraromatic fused rings.
Tag Archive: Cascade
Aristolactams: One-pot Miyaura-Suzuki/Aldol cascade microwave method
Reaction(s) of the week — Overman/RCM/Kharasch – bicyclic lactams in a microwave
Reaction of the week: Overman/Metathesis/Kharasch
Went looking for an example where the conditions of the microwave temperature can be controlled in a different way or the next step in a sequence could occur. And behold — (I have to say there is more more art that goes into organic synthesis than people truly give credit…experimental indeed). Before we get to the good stuff, some housekeeping:
Overman rearrangement: A [3,3] sigmatropic rearrangment (claisen) to provide allylic trichloacetamides through the imidate in a diastereoselective manner. Voila, here is the heat thing again — can you see me run to the lab?
Ring closing Metathesis: OK, I don’t think I need to say too much here
Cyclic version of the Kharasch addition: addition of a polyhalogenated alkane to an alkene through an atom radical transfer. In nearly all of the cyclic versions it is a metal-mediated event (used to be Cu but now Ru is much more common).
So when we see all of them laid out it seems something easy enough to combine, right? The Kharasch addition is perfectly set in place from the Overman rearrangement — and generally speaking if the olefin is in the right place, a metathesis is a much more facile process than the K cyclization.
A very elegant report out of the University of Glasgow (Tetrahedron Letters 2011) illustrates the combination of all 3 three reactions for the construction of bicyclic gamma lactams. Quickly looking at the thermal version of the scheme, it is clear that all reactions can be take a little fire under the butt to get going 18 h Overman (actually down from some of the traditional methods), 1 h Metathesis and 2-4 h Kharasch.
Without going into tandem catalysis mechanisms, be they auto or orthogonal, several things have to be kept in mind while moving distinct transformations from traditional methods to microwave irradiation such as solvent, temperature, catalyst (I think you have heard me say before — now microwave technology has caught up a bit more to allow the ability to do some reaction screening in a single operation with SRC configurations, but there was some development that went into saying this is a great microwave approach to a 3 step method to the fused lactams.
An example of what I was talking about — there have been reports where an asymmetric Overman/RCM has been tried and failed because the catalyst did not survive the reaction conditions. On the other hand, a Grubbs catalyst has been shown to perform the RCM and survive the K ring closure with higher heating. So the crux of this article hinges on finding out what solvents and what catalyst can be used or added for each step for microwave irradiation.
First optimization: Concentrating on the Overman/RCM — changing from a Pd based system to K2CO3 in toluene with heat provided the acetamide, but toluene is not the best solvent for microwave absorption, even with some added ionic contribution of the K2CO3. Adding co-solvent to boost the rate resulted in decomposition and stopping the reaction, either by coordination to the Grubbs catalyst or decomposition.
2nd optimization: Quick study revealed that the microwave conditions for the RCM could simply be used and modified to ramp up to a higher temperature following the ring closure or stopping the run, adding 4 angstrom sieves and heating to a higher temperature — most likely it could simply be ramped up and the microwave run continued with the right software.
So after screening solvents — toluene was settled as the best choice because it could be used for all three steps. For this, the group used a passive heating element (or susceptor as the technical term) in the form of a SiC ball or Carbon in a teflon sheeth in the toluene to help the reaction absorb the power necessary to get to temperature. Now the sequence is Overman in toluene — then add Grubbs catalyst apply microwave at 60C for 30 min-hr and lastly increase temperature to 180C in mw for the cyclization. All in all a pretty slick process.

Final form Overman 180C toluene (1h), Grubbs RCM 60C (20-30 min), Kharasch 180C with sieves (30-45 min)
Expanding the scope of the reaction to include O and N analogs as well as 5,5 C ring systems is illustrated in the paper as well. I have simply reduced the following table to summarize their work for analog development and a comparison of the traditional approach to the microwave approach — provides a telling picture of the advantages.
Enjoy the article or hope you enjoyed the summary of a nice microwave application!