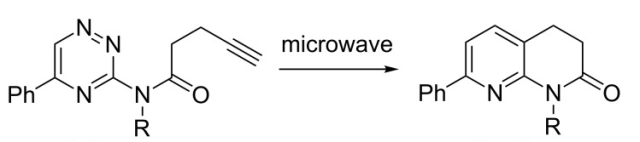
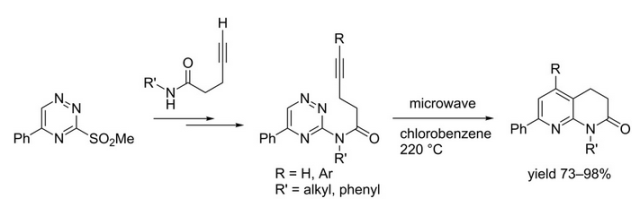
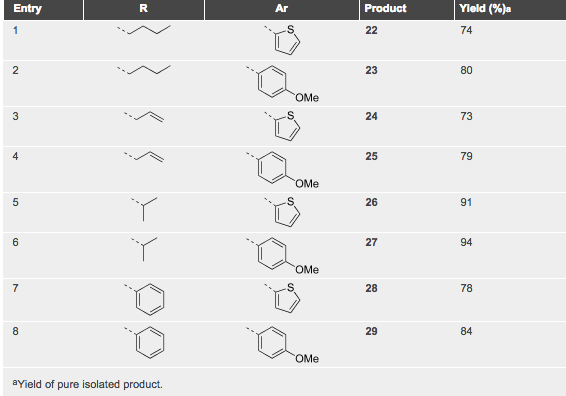
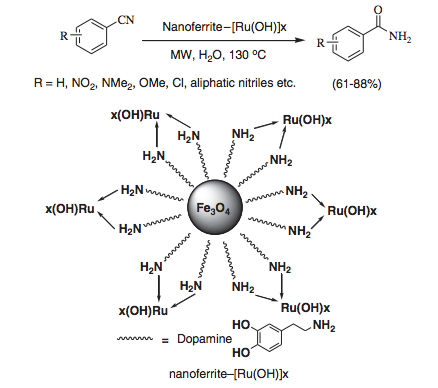
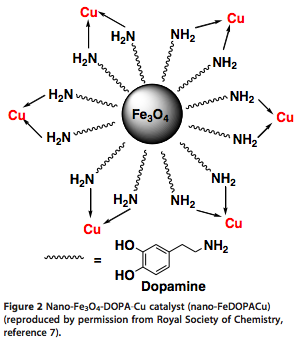
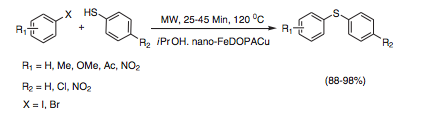
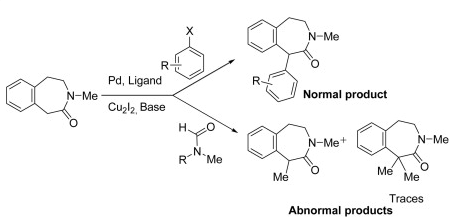
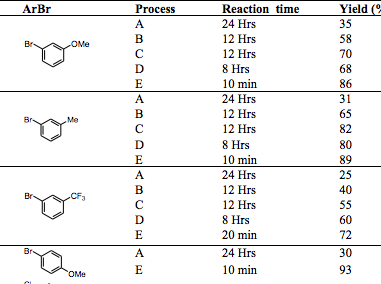
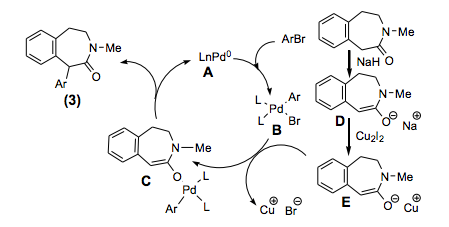
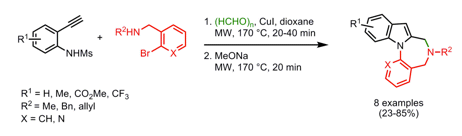
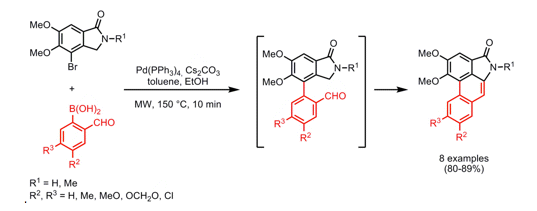
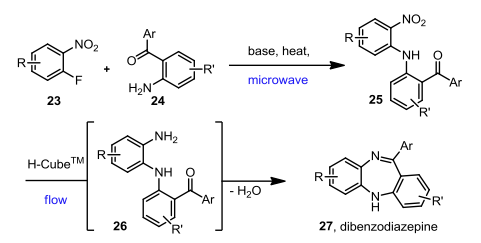
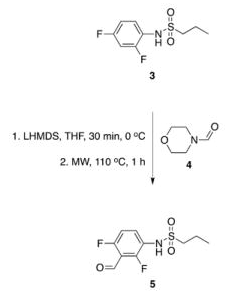
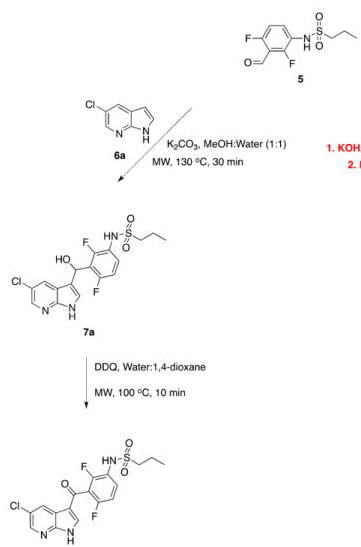
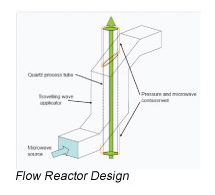
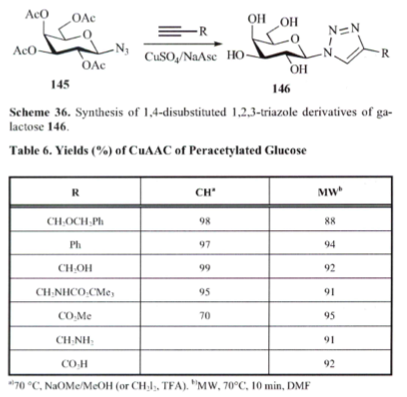
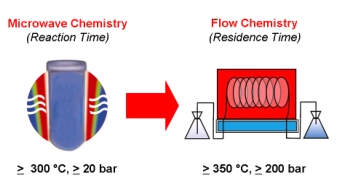
With so many labs utilizing microwave chemistry for their drug discovery processes, the effective ability to monitor the temperature of a reaction (solvent) is critical. Since each commercial microwave vessel will have its’ own set of specifications will have limitations to the temperature that can be reached without generating a significant amount of pressure, and perhaps even more critical cause an unsafe reaction and damage equipment.
Examples of commonly used solvents and their temperature and vapor pressure set at 25 bar:
Hexane: 226C
CH2Cl2 172C
Toluene 281C
EtOH 191C
Dioxane 256C
Acetonitile 218C
I set the pressure at 25 bar to illustrate that depending on the solvent, very different conditions would be used in a chemical synthesis and while may here are above the temperature normally set, often the amount of pressure held within a microwave vessel is lower. I have often found these temperatures have been used and resulted in complications to a microwave and the chemistry.
While there have been a number of reports using IR or FO temperature monitoring, it depends on the reaction being used, how much agitation, and the type of vessel material — that’s a lot to keep track of in addition to the chemistry itself. Fortunately, there are set-ups which take into account each of these key features for our benefit. I am going to outline a portion of Oliver Kappe’s presentation at EUCHEM 2012, where he spoke on each of these. His concentration in the lecture was on ionic liquid chemistry and included both microwave and flow methodology for the synthesis and use of ILs.
Back to the question of effective monitoring: I will illustrate using the Anton Paar dual temperature control on the Monowave 300, because this system provides a number of options to control the temp/power of a reaction.
The first slide indicates that effective agitation using an IR sensor plays a key role in the temperature being measured in a reaction and that FO monitoring is somewhat less critical to stirring as it is in the reaction medium being measured. One thing to keep in mind throughout the slides is that these will be using ILs, and as a highly absorbing material the set temp can easily be overshot during the course of the reaction. As we see in the graph below, with the power delivered in this set-up, the actual temperature reached is 120C over the set temp (WOW!), and depending on what is being measured, a dramatic difference in temperature is measured (in this example, you can see both biotage and CEM utilize different IR sensor engineering for their measurements.
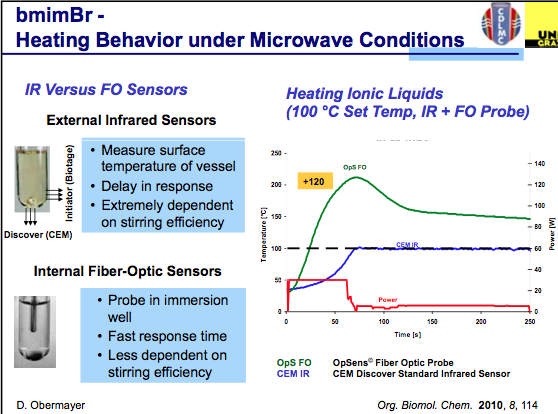
Simultaneous measurement can help improve the control of the chemistry being used. As shown in the slide below, measurements of the internal and external temperatures are be monitored and feedback to adjust the power — in this example the master slave control was set to each independently with an overshoot in the temperature internally when IR control is used…again notice the difference in the outcome for ILs. I should mention that the studies used here can be found in Org Biomol Chem 2011.
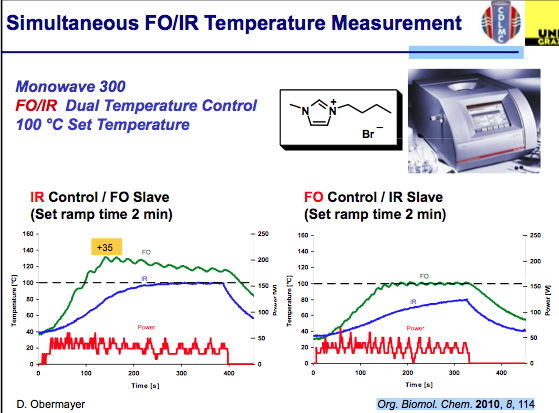
ILs are still reasonably new and have been used sparingly compared with the solvents utilized in most synthetic transformations but the field is growing. Taking a look deltaTan values listed below, we can see that ILs are a bit off the normal functioning organic and aqueous environment with absorptivity that rivals metal catalysts (and often we see the increased reaction rates of organic reactions, but can’t measure the surface of the heterogeneous metal on the surface — perhaps this is why the inorganic chemists benefit from high reaction rates at higher temperatures on the surface of the nucleation and the aqueous or organic media temperature is being measured). It takes very little time for the temperature to go beyond the set point here (notice that no more power is being delivered). The good news is that ILs have very little vapor pressure associated with them, so an overpressure here will not be an issue — but depending on your chemistry, be aware of going to extreme temperatures if you don’t have the appropriate vessel materials. So where is the solution for using ILs and being comfortable with the temperature of the reaction?
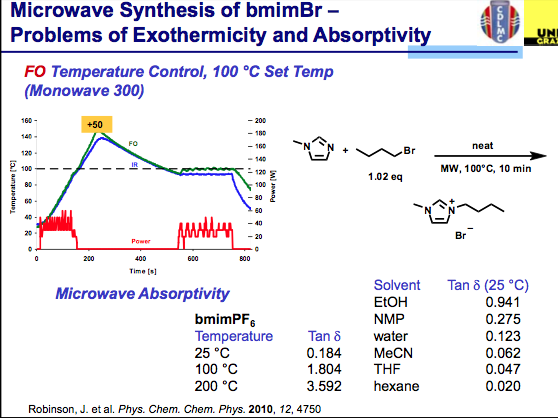
The answer is found in the same way we circumvent the ability of a microwave to be used with solvents that do not absorb microwave energy….by the appropriate choice of vessel material. You see when we use a hydrocarbon solvent or something like THF, the microwave simply delivers energy without getting the solvent up to temperature so we need to add a co-solvent (there are some tricks to this) or a susceptor so that there is something in the reaction absorbing the microwave energy and converting it into heat. Depending on the company, a version of Si-Carbide or Teflon impregnated with Carbon can be used to absorb microwave energy and not interfere with the reaction. Furthermore, their absorption properties outweigh that of the IL, so let’s take a look at the result. As we see here, pyrex shows the same issue of the lack of temperature control in the bmimBr synthesis, whereas the Si-Carbide vial absorbs the energy in a more controlled way, allowing for the reaction and temperature to stay on track with the reaction parameters.
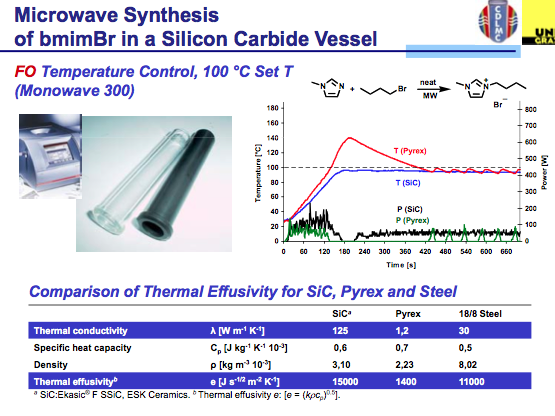
Hopefully this will help shed some light on temperature (and pressures generated) control in a single-mode microwave reaction. Certainly the ability to monitor both with control is something Anton Paar has built into their system for chemists to depend on their chemical environment and the use of Si-Carbide vials go along way in building in a way to help chemists move forward into different areas of research. Hope you enjoyed the slides (I thank Oliver Kappe for continued progress in the development of this field of research and development, and always looking for solutions to microwave development).